In an effort to improve Clinical Trials reporting in IBISResearch - IRB, a new Clinical Trials activity will be added to all study workspaces to help streamline clinical trial data capture on the NCT#, study duration, enrollment dates, and sample size (see screenshot below).
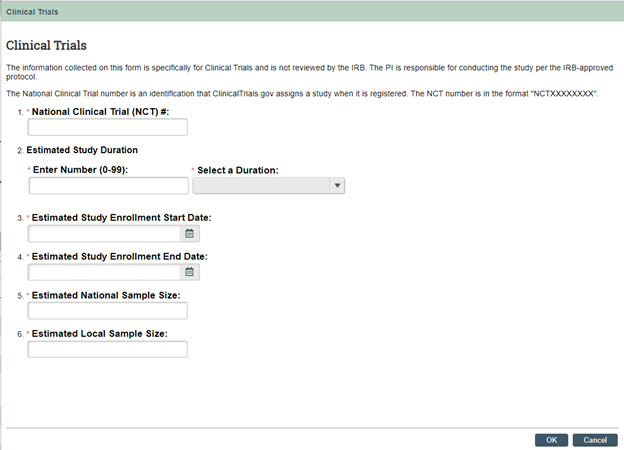
The IRB/Velos integration will be updated in the coming weeks to allow for a seamless flow of information from IRB to Velos. This clinical trial data will be entered in IRB through the new activity, instead of in Velos.
Once the data is populated in IRB, it will no longer be editable in Velos. Future changes to the existing information in Velos will need to occur in the IRB Clinical Trials activity. This updated integration will not impact the data that is currently in Velos, as existing data will only be overwritten if/when information is added in the IRB Clinical Trials activity.
The existing Velos data will not be backfilled into IRB and thus this new process will apply to new studies and studies that need to modify their clinical trial information.
Kindly note:
- This new activity must be completed for all Clinical Trials upon submission to the IRB. However, this activity will not be reviewed by the IRB nor impact the IRB review process.
- The activity will be available in all system states and can be accessed/edited by the PI, study team, committee reviewers, and ancillary reviewers.
- All fields in the activity will be required.
- The activity will retain all field data and can be edited as needed if protocol information changes.
Should you have any questions regarding this change, please reach out to OVPRShelpdesk@miami.edu.
Thank you, as always, for your continued support!