|
The Office of the Vice Provost for Research & Scholarship gathers all the research events posted to our listservs into a once-weekly email, and we also feature them on our new online calendar. Check out our primer on how to promote your event or email vprs@miami.edu for help.
|
|
|
Don't Miss it! Complete by August 31, 2023
The deadline for current staff to complete the Research Administration at the U (RAU) Curriculum is fast approaching. The deadline is August 31, 2023.
All staff who are responsible for pre- and post-award activities related to sponsored programs are required to complete the mandatory curriculum by August 31, 2023. If one or more courses are not completed, your access to IBIS:Research (Grants & Agreements) and Workday role will be suspended until the courses are complete.
This is your final opportunity to sign up for the RAU Curriculum and complete the mandatory training by the deadline date. Classes are scheduled in May, June, July, and August. The prerequisites are being offered in June. If you have pre-requisites pending and cannot view upcoming classes or register, please contact Sofia Aymerich @ s.aymerich@miami.edu. Register for the curriculum in ULearn.
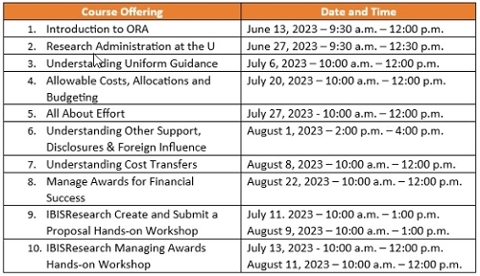
RAU was developed to ensure administrators have the tools required to support our faculty’s research efforts while ensuring compliance with regulations and policies. For more information about the RAU curriculum visit the ORA website.
|
|
|
|
|
Tuesday, June 13, 9:30 a.m. - 12:00 p.m.
This class is part of the RAU Curriculum. It provides a fundamental overview of the Office of Research Administration, sponsored policies, process and services. If you would like to get credit for this class within the RAU curriculum, please register to the RAU Curriculum in ULearn first and access this class through the Curriculum. It is important to complete this class in the order that the Curriculum is set up. Register in ULearn.
|
|
|
|
|
Thursday, June 15, 12:00 p.m. - 1:00 p.m.
Lunch with Laura is a monthly discussion session with Laura Kozma, Associate Vice President for Research Administration. There will be new focus each month, including hot topics in research administration, new and significant policies, and changes in the Office of Research Administration (ORA).
June 2023: Effort Reporting Deadlines and Salary Management
September 2023: F&A Rate Negotiation: The Space Survey
October 2023: Partnering with Research Administration to Expedite Clinical Trials
Registration is required. Register through Zoom for one or all sessions.
|
|
|
|
|
Thursday, June 15, 1:30 p.m. - 2:30 p.m.
Join a seminar hosted by the SCCC Onco-Genomics Shared Resource, SCCC Biostatistics and Bioinformatics Shared Resource and HIHG Center for Genome Technology for an in-person or virtual seminar. Presented by Kevin Shianna, Ph.D., Director, Sequencing Specialists, Illumina, Inc.
Illumina’s newly released, innovative high-throughput sequencer, the NovaSeq X+, has arrived at the University of Miami (UM). The Illumina NovaSeq X+ provides uniquely fast and cost-effective high quality next generation sequencing (NGS), creating a paradigm shift in affordable access to massive sequencing data generation. Please join us for this presentation on the possibilities that the NovaSeq X+ opens for genomics research. We will review the NovaSeq X+ technology advancements and how emerging applications on this new instrument can drive research and biological insight, including the highly anticipated Illumina Complete Long Read future application. UM is among the first sites worldwide to offer the NovaSeq X+. Working collaboratively, the Sylvester Comprehensive Cancer Center (SCCC) Onco-Genomics Shared Resource (OGSR) and the Hussman Institute for Human Genomics (HIHG) Center for Genome Technology (CGT) each recently installed a NovaSeq X+. These cutting-edge sequencing instruments are now available to support your research.
Register in advance to attend virtually or in-person at the Soffer Clinical Research Center, 1st Floor, Gordon Auditorium.
|
|
|
|
|
Wednesday, Jun 21, 10:00 a.m. - 12:00 p.m.
This class is intended for individuals who have the role of Effort Coordinator in the Employee Compensation Compliance System (ECC). This session will cover the responsibilities of the effort coordinator in the ECC System.
This will be offered as a Zoom Meeting.
Registration is through ULearn.
Key word ECC
|
|
|
|
|
Friday, Jun 23, 1:30 p.m. - 3:30 p.m.
This class is intended for individuals who have the role of Effort Coordinator in the Employee Compensation Compliance System (ECC). This session will cover the responsibilities of the effort coordinator in the ECC System.
This will be offered as a Zoom Meeting.
Registration is through ULearn.
Key word ECC
|
|
|
|
|
Tuesday, June 27, 9:30 a.m. - 12:30 p.m.
This class is part of the RAU Curriculum.
This class provides a fundamental overview of research administration at the University of Miami from proposal submission through award close and includes basic terminology, regulatory compliance, award negotiation, setup, reporting obligations, audits, and overall award management.
Please register to the RAU Curriculum in ULearn first and access this class through the Curriculum. It is important to complete this class in the order that the Curriculum is set up. If you would like to take this class as a standalone course, please contact vprs@miami.edu.
|
|
|
|
|
Wednesday, Jun 28, 10:00 a.m. - 12:00 p.m.
This class is intended for individuals who have the role of Effort Coordinator in the Employee Compensation Compliance System (ECC). This session will cover the responsibilities of the effort coordinator in the ECC System.
This will be offered as a Zoom Meeting.
Registration is through ULearn.
Key word ECC
|
|
|
|
|
Thursday & Friday, June 29-30, 9:00 a.m. - 4:30 p.m.
Scientific Computing Workshops help researchers get their work done in less time and with less pain by teaching them basic research computing skills. This hands-on workshop will cover basic concepts and tools, including program design, version control, data management, and task automation. Participants will be encouraged to help one another and to apply what they have learned to their own research problems.
It will be held at Miami Clinical and Translational Science Institute, Don Soffer Clinical Research Center, Rooms 710 R&Q on June 29 & 30. For more information visit the website or email tnorris@miami.edu.
|
|
|
|
|
Friday, Jun 30, 1:30 p.m. - 3:30 p.m.
This class is intended for individuals who have the role of Effort Coordinator in the Employee Compensation Compliance System (ECC). This session will cover the responsibilities of the effort coordinator in the ECC System.
This will be offered as a Zoom Meeting.
Registration is through ULearn.
|
|
|
|
|
Thursday, Jul 6, 10:00 a.m. - 12:00 p.m.
This Class is part of the RAU Curriculum.
This class provides an in-depth look at the Uniform Guidance for the sponsored grants and agreements.
Please register to the RAU Curriculum in ULearn first and access this CBL through the Curriculum. It is important to complete this class in the order that the Curriculum is set up. If you would like to take this class as a standalone course, please contact vprs@miami.edu.
Register in ULearn.
|
|
|
|
|
|
|
Tuesdays & Thursdays, 1:00 p.m. - 2:00 p.m.
One-on-one opportunity to connect with Human Subjects Research Office team members for questions and guidance, open to anyone involved in human subjects research at the University of Miami or the Jackson Health System. First come, first serve, no appointments necessary.
Read More >
|
|
|
|