|
In the Office of the Vice Provost for Research & Scholarship, over 250 professionals guide faculty, staff, and learners through the entire Research Lifecycle, from project conception and grant submission to sharing results with the world and creating commercial opportunities
To submit an announcement to the Week in Review, email vprs@miami.edu or to have your event appear in the Research Events Round Up newsletter promote your event.
|
|
|
Friday, February 10, 10:00 a.m. - 12:00 p.m.
The OVPRS Administrative Update meeting will be held on Friday, February 10th from 10:00 a.m. - 12:00 p.m.
Join leaders from the Office of the Vice Provost for Research + Scholarship for this update meeting which is designed to provide information to the University research community on policy, procedural and process changes related to research activities. Agenda items include:
- New Post-Award Director
- F&A Costs on Sponsored Awards
- IBISResearch Accuracy – Human Subjects & Account Setup Delays
- PG Accounts for Closed & Closing Projects in Workday
- Form H live
- IBISResearch Grant and Agreement Down Time
- Clinical Trial Fee Changes
- New Research IT Director
- Effort Certification and Cross Company PAA’s
- NSF 23-1 PAPPG - Safe and Inclusive Working Environments for Off-Campus or Off-Site Research
- Research Administration Curriculum Deadline
- NIH Data Management Plan
You have the opportunity to submit your questions prior to the meeting by clicking HERE.
Register in advance for the virtual Zoom meeting by clicking HERE.
|
|
|
Announcements & Reminders
|
|
|
|
|
Important Reminder
This is a reminder that the State of Florida has a law, passed in 2013, requiring research laboratories that order medical compressed gasses (such as liquid nitrogen and CO2) to have a medical gas exemption in place which allows these gases to be delivered to non-clinical settings without the need for a medical license. Since January 2022, these exemptions are only valid for two years and a new exemption request must be submitted with FL Department of Business and Professional Regulation (DBPR) office.
If you do not have an active exemption in place, Airgas and other vendors will likely refuse to deliver any more gases to your lab until you have a current exemption in place. Please note that the Florida State DBPR office is experiencing delays of up to 90 days for some exemption requests.
There is no cost for the exemptions and renewals. To file for the free medical gas exemption, please follow these steps:
Click on this link FL Department of Business and Professional Regulation (DBPR) office.
- Select Apply for a new license.
- Under LICENSING & REGULATIONS, select Drugs, Devices & Cosmetics.
- Go to Exemptions
- Select Online Applications and fill out the Exemption Letter - Application Summary.
- Ensure that the address you file matches your laboratory, not an office.
- When asked for the vendor number, enter the Airgas vendor number which is 31170 or other vendor number of your choice.
To avoid a disruption in your gas deliveries, send your current exemption to Airgas or other vendor for their files.
For more information, please contact Environmental Health and Safety at (305) 243-3400.
|
|
|
|
|
Attention University Faculty Researchers
In an effort to provide added value to you and your research, we would like to gather your feedback about what information technology (IT) services or tools you would be interested in to assist with your research. Your responses will help us evaluate and implement solutions to assist our community in future research projects. This survey should take no more than 2-minutes to complete, and responses are anonymous. Take the survey here.
|
|
|
|
|
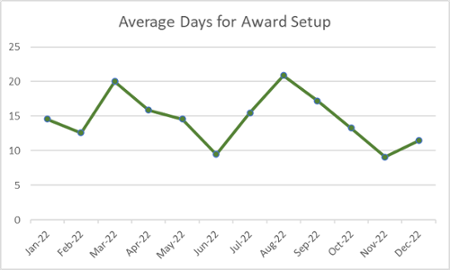
Monthly Metrics
Research Administration continues to provide monthly metrics for services provided to the University research community. Here are the metrics for December 2022.
Award Setup
19 new awards setup
358 amendments, adjustments and corrections processed
3 awards pending setup for 30 days or more
Contracts (excluding clinical trials)
222 contracts executed
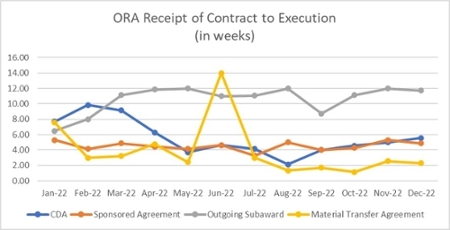
The times here include all aspects of the process once the agreement is routed to ORA:
- Review of the terms
- Negotiation
- Review by other UM offices when required (Office of Technology Transfer, Office of General Counsel, etc.)
- Sponsor review time
- Signing
|
|
|
|
|
Now Available!
Based on feedback regarding the process for Payroll Accounting Adjustments (PAAs), Enterprise Business Solutions (EBS), in collaboration with the Office of Research Administration (ORA), has created a step-by-step guide on how to properly submit a PAA in Workday.
Highlights of this guide include:
- Troubleshooting Workday PAAs with Multi-Driver worktags
- Selecting the correct related worktags
- Selecting the correct Company for the PAA
You can access this guide by clicking here or via the Workday Informational website.
For all inquiries, please contact the EBS team at workday@miami.edu or the ORA team by emailing Gloria Gari at ggari@miami.edu.
|
|
|
|
|
Executive Level II Salary Increased
The Office of Personnel Management recently released new salary levels for the Executive Pay Scale. Effective January 1, 2023, the salary limitation for Executive Level II is $212,100.
For issued awards that were restricted to Executive Level II (see historical record of salary cap link below), including competing awards already issued in FY 2023, if adequate funds are available in active awards, and if the salary cap increase is consistent with the institutional base salary, recipients may rebudget funds to accommodate the current Executive Level II salary level.
For a historical record of the salary cap, including effective dates, see visit the NIH website.
|
|
|
Important NIH Notices
- Forms-H Coming for January 23, 2023 Due Dates. Applicants applying to NIH funding opportunities with due dates on or after January 25, 2023 must use updated application forms and instructions identified with a Competition ID of “FORMS-H” (NOT-OD-22-195). The primary change to the updated application forms is the addition of an “Other Plan(s)” attachment on the PHS 398 Research Plan and PHS 398 Career Development Supplemental forms as part of the implementation of the 2023 NIH Data Management and Sharing Policy. Applicants must attach the required Data Management and Sharing Plan in this new field in FORMS-H applications (NOT-OD-22-189).
- Notice for the NIGMS Grant Writing Webinar Series for Institutions Building Research and Research Training Capacity The National Institute of General Medical Sciences (NIGMS) will host an informational webinar series for faculty and sponsored programs/research development personnel from institutions building research and research training capacity. During the webinars, suggestions will be shared for navigating the process of seeking NIH funding. Attendees will learn considerations for determining research idea and grant writing readiness, selecting opportunities to apply for, effectively writing a grant application, and seeking appropriate feedback. Registration is required to attend.
- Do you have questions about the National Institutes of Health (NIH) Inclusion Plans? In Part 1 of this NIH All About Grants podcast mini-series, NIH's Inclusion Policy Officer, Dawn Corbett, discusses how to consider inclusion plans when putting together your application (MP3 / Transcript). Part 2 of the podcast covers inclusion plans during peer-review and post-award (MP3 / Transcript). NIH's All About Grants episodes can also be heard on iTunes and Spotify. Read the article in the Extramural Nexus.
- Updated Requirements for NIH Notification of Removal or Disciplinary Action Involving Program Directors/Princial Investigators or other Senior/Key Personnel. Section 239 now requires that, “[t]he Director of the National Institutes of Health shall hereafter require institutions that receive funds through a grant or cooperative agreement during fiscal year 2022 and in future years to notify the Director when individuals identified as a principal investigator or as key personnel in an NIH notice of award are removed from their position or are otherwise disciplined due to concerns about harassment, bullying, retaliation, or hostile working conditions.” Therefore, effective 60 days from the publication of this Notice, NIH recipient institutions are required to notify NIH when individuals identified as PD/PI or other Senior/Key personnel in an NIH notice of award are removed from their position or are otherwise disciplined by the recipient institution due to concerns about harassment, bullying, retaliation or hostile working conditions. Notification must be provided by the Authorized Organization Representative within 30 days of the removal or disciplinary action and must be submitted to NIH through a dedicated web form.
- NIH NIAID Technology Transfer Fellowship Program. Fellows will be mentored by professionals that work side-by-side with with world-renowned NIAID and Centers for Disease Control and Prevention (CDC) scientists and will be part of the team that helps transfer innovations from the lab to commercial products (including vaccines, therapeutics, and diagnostics) that benefit global public health.
- NIH Grants Policy Statement (Rev. Dec 2021) This update is applicable to all NIH grants and cooperative agreements with budget periods beginning on or after October 1, 2021. This update supersedes, in its entirety, the NIHGPS dated April 2021. Previous versions of the NIHGPS remain applicable as standard terms and conditions of award for all NIH grants and cooperative agreements with budget periods that began prior to October 1, 2021. This update incorporates new and modified requirements, clarifies certain policies, and implements changes in statutes, regulations, and policies that have been implemented through appropriate legal and/or policy processes since the previous version of the NIHGPS dated April 2021.
- Childcare Funding Available for Pre/Post Docs Full-time appointed predoctoral and postdoctoral NIH-NRSA supported trainees are eligible to receive $2,500 per budget period for childcare costs provided by a licensed childcare provider. For households where both parents are eligible full-time predoctoral or postdoctoral NRSA trainees, each parent is eligible to receive $2,500.
- Guidance on Salary Limitation for Grants and Cooperative Agreements This Notice provides information regarding the salary limitation for NIH grant and cooperative agreement awards and extramural research and development contract awards.Since 1990, Congress has legislatively mandated a limitation on direct salary for individuals under NIH grant and cooperative agreement awards (referred to here as a grant). The mandate appears in the Consolidated Appropriations Act, 2023 (Public Law 117-328), signed into law on December 29, 2022, which provides authority for NIH to incur obligations for Fiscal Year 2023. The Consolidated Appropriations Act, 2023 restricts the amount of direct salary to Executive Level II of the Federal Executive pay scale. The Office of Personnel Management recently released new salary levels for the Executive Pay Scale. Effective January 1, 2023, the salary limitation for Executive Level II is $212,100.
|
|
|
|