Environmental Health & Safety from the Director's Desk
|
|
|
Hello everyone,
Welcome to the beginning of another school year here at the U. As we move towards the first day of class, we are only part way through our summer in Miami. This quarter’s newsletter includes some helpful reminders on hot weather guidance. August is a challenging month for those of us that live here and for those moving here for the first time.
A big thank you to those who attended our first annual Laboratory Safety Awareness Week webinars this past May! It was an exciting week for us and hopefully an educational and helpful week for you. Our good news is that the custom University of Miami Laboratory Research Notebooks have arrived. We have begun delivery to those that won them through their Laboratory Safety Awareness Week attendance, as you can see in the picture below.
Best of luck to everyone with the new semester starting and new students to acclimate to your laboratories. Feel free to reach out to our team for assistance in making this year smooth, productive and, of course, safe
|
|
|

|
|
Warm regards,
Jennifer Laine, DrPH
Executive Director, EHS
|
|
|
|
Staying Safe in Hot Weather: Tips to Prevent Heat-related Illnesses
Heat represents a significant risk to public health across our country and climate projections indicate that extreme heat events will be more frequent and intense in coming decades. With this record-breaking summer in South Florida, our exposure to intense sun, elevated temperatures, and humidity is higher and our best way to overcoming heat-related illnesses is through prevention.
The amount of heat a person can tolerate depends on several factors, including exposure duration, age (with children and older adults being at higher risk), physical health, hydration status, and recent heat exposure.
Heat-related illnesses occur when the body’s core temperature rises above healthy levels. This can happen when the body heats up too quickly, preventing proper cooling, or when excessive fluid or salt is lost due to dehydration or sweating, compromising normal bodily functions.
Here are some safety tips to prevent heat-related illnesses:
• Be informed and stay alert: pay close attention to heat advisories or warnings issued for your area, you can find accurate weather alerts at: http://www.nws.noaa.gov/
• Prepare for hot weather or extreme heat: smart choices can significantly impact your well-being during temperature surges:
- Apply high-SPF sunscreen frequently.
- Schedule work or strenuous activities in early morning or late evening.
- Opt for lightweight, light colored, non-restrictive clothing.
- Stay hydrated – drink plenty of water or other nonalcoholic fluids.
- Eat light, easy-to-digest foods.
- Seek out shade and schedule breaks during extended outdoor periods.
- Spend more time in air-conditioned places.
• Know the warning signs and symptoms of heat-related illnesses:
- Mild heat illnesses include heat rash, which occurs when sweat cannot evaporate from the skin; muscle cramps, which are painful muscles spams that can appear immediately or hours after the exposure; and heat syncope, dizziness, lightheaded feeling or even a fainting episode after standing too long or getting up suddenly.
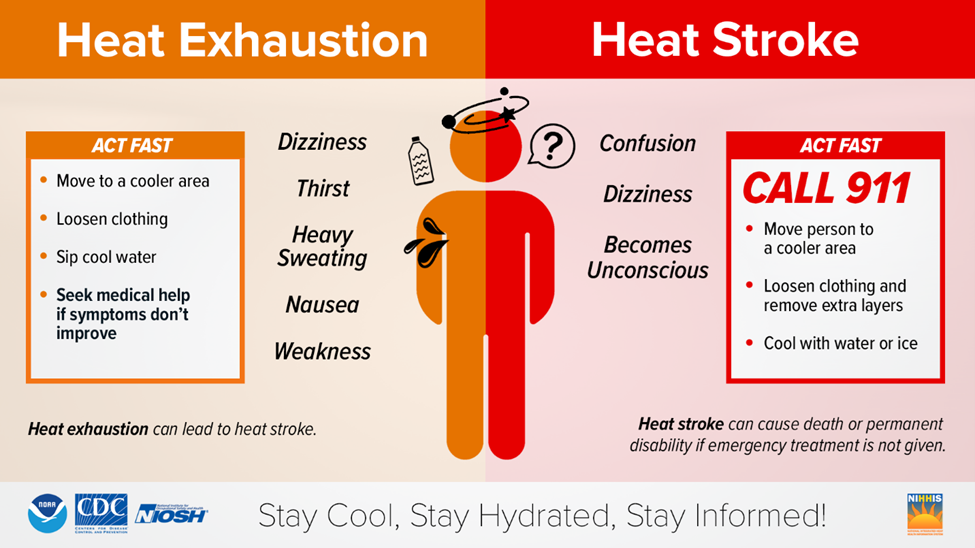
- Severe heat illnesses include heat exhaustion with symptoms that include headache, nausea, vomiting, heavy sweating, weakness, cool, and clammy skin, weak pulse, and normal temperature; and heat stroke (severe emergency), which requires immediate medical attention. Symptoms include throbbing headache, confusion, nausea, dizziness, high body temperature (106°F or higher), rapid and strong pulse, unconsciousness, and hot, dry skin.
• Know what to do if you or someone around you experiences heat-related symptoms:
- Heat exhaustion or mild systems: get into a cool environment and hydrate - quickly. Loosen clothing and fan yourself or move to an air-conditioned area. Use a water mister to reduce body temperature. Take sips of water; if nausea occurs, discontinue water intake. For muscles cramps, apply firm pressure to cramping muscles or gently massage to relieve spasms.
- Heat stroke (severe emergency), take steps to get the individual to a hospital immediately, including summon emergency medical assistance. Delay in this case can be fatal.
Remember, when working or spending time outdoors, it’s essential to plan ahead, stay cool, and hydrated. For a quick review of heat exhaustion versus heat stroke, refer to the graphic below:
|
|
|
|
|
If you have any questions, please contact Noelia Estevez de Rosario.
|
|
|
|
Avoid Leaving Unwanted Items in the Corridor
Removing unwanted items from building corridors is essential for safety, accessibility, and aesthetics. Cluttered corridors can impede emergency evacuations, hinder maintenance tasks, and create an unwelcoming environment.
The Surplus Property Department manages requests for disposing of university owned items across our campuses and satellite locations.
To request the disposal of surplus items like furniture, cabinets, microscopes, or other laboratory equipment, complete the surplus transfer form. For data storage devices (computers, laptops, PCs), use the e-waste surplus transfer form. Once completed, email the forms to surplusproperty@miami.edu to schedule a pickup.
Use the surplus transfer form for discarded office furniture and laboratory equipment:
|
|
|
|
Use the e-waste surplus transfer form for discarded data storage devices:
|
|
|
Let’s all work together to keep the corridors free and clear of obstructions. A well-organized corridor contributes to a safer and more pleasant building environment, enhancing the overall experience.
If you have any questions, please contact Christine Daley.
|
|
|
|
Overfilled Containers are a Common Issue in Biomedical Waste Management
Properly managing biomedical waste containers is crucial to prevent exposure, contamination and maintain safety. Overfilling containers can result in spills or exposure to hazardous waste. This is a particular concern that jeopardizes safety since it is often difficult to identify specific waste and what hazard it may have contained, making treatment to exposure a challenge. The overfilled containers also can affect our disposal procedures.
Note that other personnel, perhaps non-lab personnel, may be in the chain of handling waste. Overfilling makes it difficult to move as it can be too heavy or awkward to carry. This increases that chance that others can be exposed to the hazardous waste as well. Keep this in mind as you think of your own safety as well as to the safety of others that support your lab!
Sometimes lab personnel try to compact the waste thinking it is a more efficient use of disposal bags and space. However, this approach only increases the risk of spills and exposure. Please do not compact your hazardous waste!
These pictures are examples of overfilled waste containers.
|
|
|

|
|
Here are some tips for managing your biomedical waste safely:
• When a waste container reaches 80% full, treat the container as full.
• Allow enough room to properly close the container.
• Review waste containers at the end of the day and dispose of full containers.
• Ensure that there are enough spare containers and bags available.
• Change out biomedical waste containers every 30 days.
• Transfer wastes from an overly full container to an empty container; do not attempt to compress the waste to fit.
Remain vigilant about your proper waste disposal practices. Regularly monitoring waste containers and promptly addressing overfilling can prevent contamination and minimize risks. Additionally, emphasizing safety protocols during laboratory training and meetings can help raise awareness about the importance of responsible biomedical waste management.
You can find a table with vendor and product information for compliant biomedical waste containers and bags on the EHS website at Hazardous Materials | Environmental Health and Safety | University of Miami. If you have any questions, please contact Brian Cumbie.
|
|
|
|
New Training Options in ULearn
To better serve our clinical researchers, we’ve added our training suite to ULearn. The Biosafety Training webpage has been updated to reflect these new offerings, with direct links to each of them. These new trainings include:
• General Biosafety (every 3 years)
• Biosafety for Clinicians (every 3 years)
• Recombinant DNA Training (every 3 years)
• Laboratory Safety (every 5 years)
• Basic Laser Safety (every 3 years)
• Shipping of Dangerous Goods (every 2 years)
• Shipping of Biological Materials (every 2 years)
For non-clinical researchers, you can still take all our trainings on Blackboard Ultra, with General Biosafety being offered on Elevate as well. And as always, you can take any of our trainings directly with us by webinar. Webinar based trainings are offered on a set schedule (see our website for more details). We are also happy to offer private sessions by request.
Join the Festivities of Biosafety Month This October
Every year we recognize and celebrate Biosafety and Biosecurity Month in October. Since 2020, we’ve hosted webinars highlighting major projects aimed at promoting a safety culture here at the U. Last year we stepped up the festivities by offering free food during lunch and learns, along with prizes for attendees at our annual webinar. As we plan for this year’s Biosafety Month, keep an eye out for event notifications and attend as many as you can. We’ll also post our plans on the Biosafety webpage as they come together.
|
|
|

|
|
If there are any questions, please contact us at biosafety@miami.edu
|
|
|
|
Peroxide-forming Chemicals (PFCs) in Laboratories
Peroxide-forming chemicals (PFCs) are compounds that can spontaneously form shock-sensitive explosive peroxide crystals. These peroxides pose significant risks to laboratory safety.
Hazards of Peroxide Formation
PFCs can react with oxygen in an auto-oxidation process, leading to peroxide formation. Once peroxides are present, these chemicals become highly sensitive to thermal or mechanical shock, potentially resulting in violent explosions. Common PFCs include ethers, such as diethyl ether and tetrahydrofuran, as well as other solvents like dioxane and acetone.
Best Practices for Safe Handling and Storage
To minimize the risks associated with PFCs, follow these best practices:
- Storage Containers: Store all peroxidizable compounds in tightly closed, air-impermeable, light-resistant containers. Use original manufacturer containers whenever possible. Replace caps promptly after use to prevent peroxide formation.
- Avoid Light and Heat: Keep PFCs away from light, heat, direct sunlight, sources of ignition, oxidizers, and oxidizing agents. Peroxide formation can be accelerated by exposure to light and elevated temperatures.
- Consult SDS: Consult the Safety Data Sheet (SDS) for each chemical. Some PFCs may be safely stored under certain conditions, such as under nitrogen, to minimize oxygen exposure.
- Record Keeping: Maintain records for all PFCs, including the date of receipt and date of container’s first opening. Clearly label containers with this information.
- Inspect Containers: If a chemical’s age or history is unknown or if visible signs of peroxide formation (such as crystals, discoloration, or stratification) are present, do not move or open the container. Contact the EHS HAZMAT team for assistance.
- Rinse Empty Containers: Rinse empty containers that once held PFCs to prevent residual peroxides from concentrating.
- Avoid Volatile Loss: Be cautious with nearly empty containers where solvent loss cannot be accounted for as concentrated peroxides may be present.
Don’t forget Isopropyl Alcohol!
When it comes to pure isopropyl alcohol (IPA), it can indeed form peroxides over time. This can easily be avoided. Here are some key points:
- Formation Mechanism:
a. IPA, like other ethers and solvents, can react with oxygen in an auto-oxidation process.
b. Peroxide formation occurs due to the unstable O-O bond in peroxides, which tends to spontaneously decompose, sometimes violently.
c. Factors like friction, heat, or mechanical shock can initiate this decomposition.
- Storage and Handling:
a. Store IPA in its original, airtight bottle away from light and heat sources.
b. If transferred to a secondary container, date the container and ensure that the material is used and cleaned out on a regular basis.
c. Peroxide formation may accelerate once the container is opened.
d. Visually inspect the container for signs of peroxide formation (e.g., crystals, cloudiness, discoloration).
e. Do not open if any of these conditions are observed; contact Environmental Health and Safety (EHS) HAZMAT team immediately.
- Testing:
a. Test for peroxides using a test strip periodically or ask the HAZMAT team to do it for you.
b. Perform testing before distillation, evaporation, or any other high-hazard application.
Conclusion
In summary, proper management of PFCs is crucial to laboratory safety. By following best practices, researchers can minimize the risk of peroxide formation and ensure a safer working environment. Awareness, vigilance, record-keeping, and adherence to guidelines are essential for preventing accidents related to peroxide-forming chemicals.
If you have any questions or would like a consultation, please contact Adrian Hernandez Ferrer.
|
|
|
|
Need more information or have any questions?
|
|
|
|