Environmental Health & Safety from the Director's Desk
|
|
|
|
Greetings! As we approach the end of the year, today's newsletter provides some relevant reminders ranging from decorating to disposal.
In addition to our annual decorations policy reminder, we do have an excellent article from our Industrial Hygienist for the technical—and not so technical—oriented on the use of lithium-ion batteries.
Keep in mind that as we are back to traveling and visiting our friends and loved ones around the world, business travel is back as well. This includes regulators and inspectors! We have already seen an increase in in-person visits. If you have any questions or concerns about keeping your space compliant, feel free to reach out to the Office of Environmental Health and Safety (EHS).
Please review the newsletter details below for helpful hints. Stay safe and healthy this holiday season!
Warm regards,
Jennifer Laine, DrPH
Executive Director, EHS
|
|
|
Holiday Decorating "Do's and Don'ts"
As the holidays approach, EHS wants to remind the University community of some decorating "do's and don'ts:"
|
|
|

|
|
Do's
- Do use UL-listed decorative lights, decorations, and ornaments.
- Do use artificial holiday trees that are fire-resistant and place indoor trees away from traffic.
- Do unplug holiday lights when you leave the office for the day.
- Do remove all decorations, displays, lights, and/or ornaments after the holiday event or prior to the University's annual holiday closure.
Don'ts
- Do not block doorways or fire safety equipment!
- Do not use decorative sprays or hang decorative materials (lights, ornaments, etc.) on any fire protection equipment (fire extinguishers, sprinkler heads, smoke detectors, fire alarm pull boxes, etc.), on or near exits or emergency lights.
- Do not place decorative materials in any manner that could present a fall or trip hazard, or impede egress.
- Do not bring or burn candles, incense sticks, and/or other related devices/accessories.
For more information, contact Christine Daley at 305-243-8443 or c.daley@miami.edu.
|
|
|
|
Tips for Biomedical Wastes
Following a successful biosafety month, our team would like to reiterate proper biomedical waste management in the laboratories. Following the steps below will help lead to a safe and compliant workspace.
- Accumulate and dispose of biomedical waste in impermeable red bags that meet ASTM 1709 and ASTM 1922 standards.
- Biomedical waste containers must bear the international infectious substance label and be labeled with our building and room number, street address, and city.
- Helpful hint: EHS suggests pre-printed Avery labels with the lab information that can be placed on the bag ahead of starting use.
- There must be enough room left in a biomedical waste bag to be sealed with a strong gooseneck knot. The general rule is ¾ full is full. (See below for pictures on how to tie this knot!)
- Sharps must be placed into a sharps container.
- Leave room to close your sharps containers. Most sharps containers have a "full" line in them.
- On the Medical campus, take your full waste containers to the designated biomedical waste accumulation area on your floor.
- On the Coral Gables and RSMAES campuses, please submit a signed Chemical Waste Disposal Form to EHS, and EHS will collect and dispose of the containers.
For convenience, EHS has a list of compliant biomedical waste bags on our website from a few of our vendors. If you have any questions or would like to schedule training for your lab, please contact Brian Cumbie.
|
|
|
|
How to Properly Tie a Biohazard Bag Using a Gooseneck Knot:
|
|
|
|
Step 1:
Gather, Twist Ends 8"-10"
|
|
|
|
Step 2:
Make Loop with the Twisted End
|
|
|
|
Step 3:
Push End through Loop and Pull Knot Secure
|
|
|
|
(Optional) Step 4:
Seal Tightly with Either Duct Tape or Plastic Tie
|
|
|
Biological Hygiene Plans
If you are not already aware, every lab must electronically submit a Biological Hygiene Plan (click here to download). Completion of this vital document should be submitted with the lab Biological Registration in Scishield (formerly known as BioRAFT). If you are already outlining the materials and you're working with the projects that are tied to those materials, you may be wondering what exactly the purpose of a hygiene plan is. Here's a quick explanation:
- It is a requirement for BSL-2 labs and a best practice we observe in all biological labs here, as outlined in the Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition. As we are occasionally visited by governing bodies, or experience an emergency event, having these SOPs in place beforehand is critical for compliance and guidance.
- It provides a chance for lab management to conduct a risk assessment on the work being proposed to self-assess what risks may be present that they had not previously considered.
- It outlines the associated SOPs in safety that lab members have a right to be aware of and should know while working in the lab. There's even a section to outline the lab's own special use standard operating procedures, such as procedures that would require the recapping of needles, providing the lab with a formalized process of outlining those SOPs, and getting EHS approval for them.
- Our hygiene plan has been tailored to screen for highly regulated materials, such as Select Agents, or projects that would fall under Dual Use Research of Concern (DURC), so those labs can get the additional guidance and oversight that would be required for work of that nature.
- It gives EHS Biosafety an opportunity to review the materials and propose manipulations in context with risk mitigation strategies to ensure they are appropriate, best available practices, providing additional safety oversight.
When each section of the Biological Hygiene Plan is completed with care and consideration, the utility of this document becomes vital on multiple fronts. Keep in mind: each lab conducting biological research is only required to submit one, regardless of how many projects are ongoing in your lab. As always, if you have questions or need more specific guidance, we are always happy to help at EHS Biosafety.
|
|
|
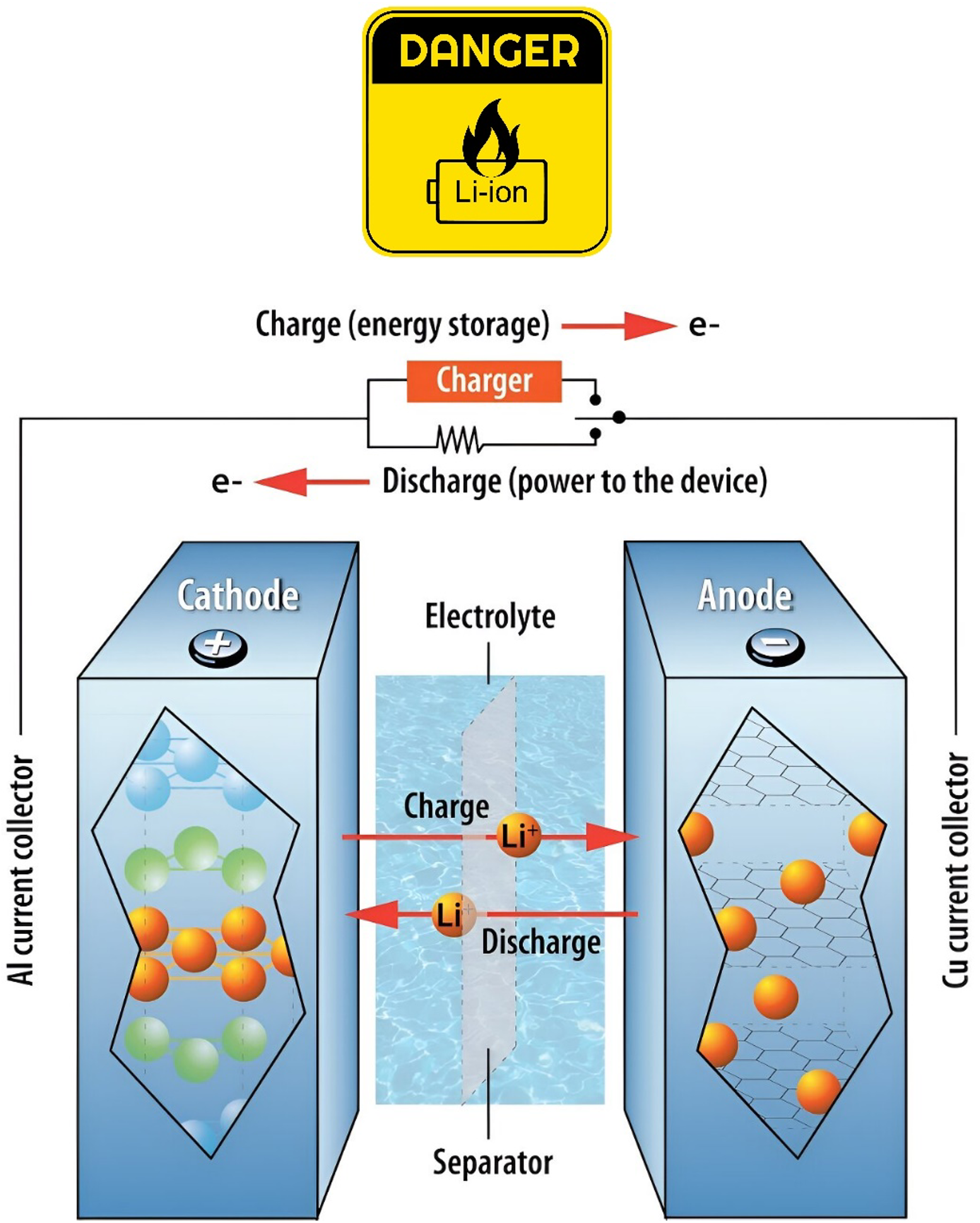
Lithium-Ion Battery Fires: A Growing Concern
On Wednesday, March 2, 2023, Spirit Airlines Flight 259 from Dallas to Orlando had to be diverted to Jacksonville due to a fire on board. Fortunately, no one died or was seriously injured, but 15 passengers and crewmembers ended up in the hospital. It was determined that the fire started in an overhead compartment after a battery from a passenger's item caught fire. What kind of battery was it? A lithium-ion battery—found in so many commonly used devices today.
Incidents such as the one described are happening in airplanes, homes, and businesses more and more frequently. At the University of Miami, we are all responsible for keeping our workplace safe, including in regard to the use of these batteries.
What causes lithium-ion batteries to spontaneously ignite?
The short answer is that lithium is the most active metal known. Under certain circumstances, when lithium comes in contact with oxygen, the two start "fighting." This happens when oxygen is generated inside the battery. When the metal oxides in the battery's cathode (positively charged electrode) get too hot, decomposition can occur resulting in a release of oxygen. Oxygen feeds the fire and keeps it going.
What can cause this to happen? Here are some examples:
- Physical damage, such as crushing or penetration.
- Electrical damage, either external or internal, resulting in a short circuit.
- Overheating, which can occur when the battery is charged too quickly or overcharged.
Are toxic chemicals released when a lithium-ion battery ignites?
Aside from the intense fire, gases are released, including hydrogen fluoride gas (HF), phosphorus pentafluoride (PF5), and phosphoryl fluoride (POF3).
|
|
|
|
|
What can we do to prevent a lithium-ion battery fire?
Here are some safety tips from the National Fire Prevention Association (NFPA) regarding lithium-ion batteries:
- Purchase and use devices that are listed by a qualified testing laboratory.
- Always follow the manufacturer's instructions.
- Only use the battery that is designed for the device.
- Put batteries in the device the right way.
- Only use the charging cord that came with the device.
- Do not charge a device near flammable or combustible liquids or materials.
- Do not keep charging the device or device battery after it is fully charged.
- Keep batteries at room temperature when possible. Do not charge them at temperatures below 32°F (0°C) or above 105°F (40°C).
- Store batteries away from anything that can catch fire.
What are the warning signs that it is time to safely dispose of a lithium-ion battery?
Bulging is probably the most obvious sign of a problem, as shown in the picture below.
If you notice an odor, change in color, overheating, leaking, or odd noises take action to properly dispose of the battery.
|
|
|

|
|
Who can I contact at EHS for assistance in removing a suspect battery from the workplace?
Contact our Hazmat team to assist you in removing and disposing of batteries safely: ehschemicalwaste@med.miami.edu.
|
|
|
|
|
Typical Chemical Hazards Present in Labs
Having hazardous chemicals in the workplace is common, especially when working in a research or academic laboratory. OSHA's Hazard Communication Standard (HCS) 29 CFR 1910.1200(c) requires that chemical manufacturers and importers classify the hazards of chemicals they produce or import and that employers provide information to their employees about the hazardous chemicals to which they are exposed by providing warning labels, safety information, and training.
Hazardous chemical means any chemical classified as a physical or health hazard, a simple asphyxiant, combustible dust, pyrophoric gas, or hazard not otherwise classified.
|
|
|
|
Chemical hazards can be classified as health hazards and/or physical hazards.
|
|
|
|
|
Health hazard means a chemical which is classified as having one of the following hazardous effects:
- Acute toxicity (any route of exposure).
- Skin corrosion or irritation.
- Serious eye damage or eye irritation.
- Respiratory or skin sensitization.
- Germ cell mutagenicity.
- Carcinogenicity.
- Reproductive toxicity.
- Specific target organ toxicity (single or repeated exposure).
- Aspiration hazard.
|
|
|
|
|
Physical hazard means a chemical that is classified as having one of the following hazardous effects:
- Explosive.
- Flammable (gases, aerosols, liquids, or solids).
- Oxidizer (liquid, solid, or gas).
- Self-reactive.
- Pyrophoric (liquid or solid).
- Self-heating.
- Organic peroxide.
- Corrosive to metal.
- Gas under pressure.
- Contact with water causes flammable gas emission.
|
|
|
|